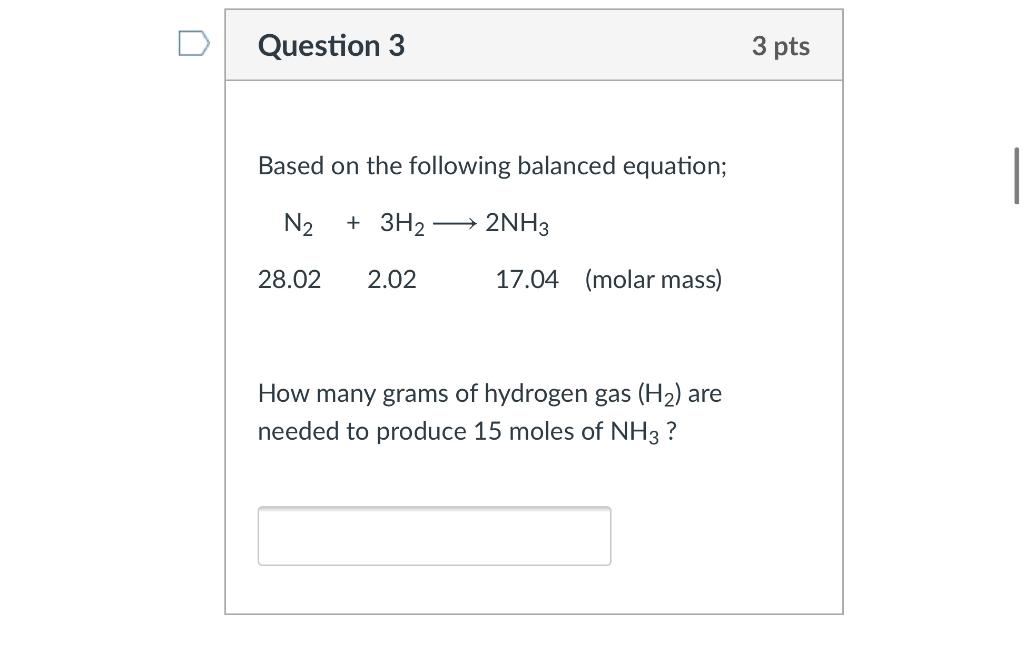
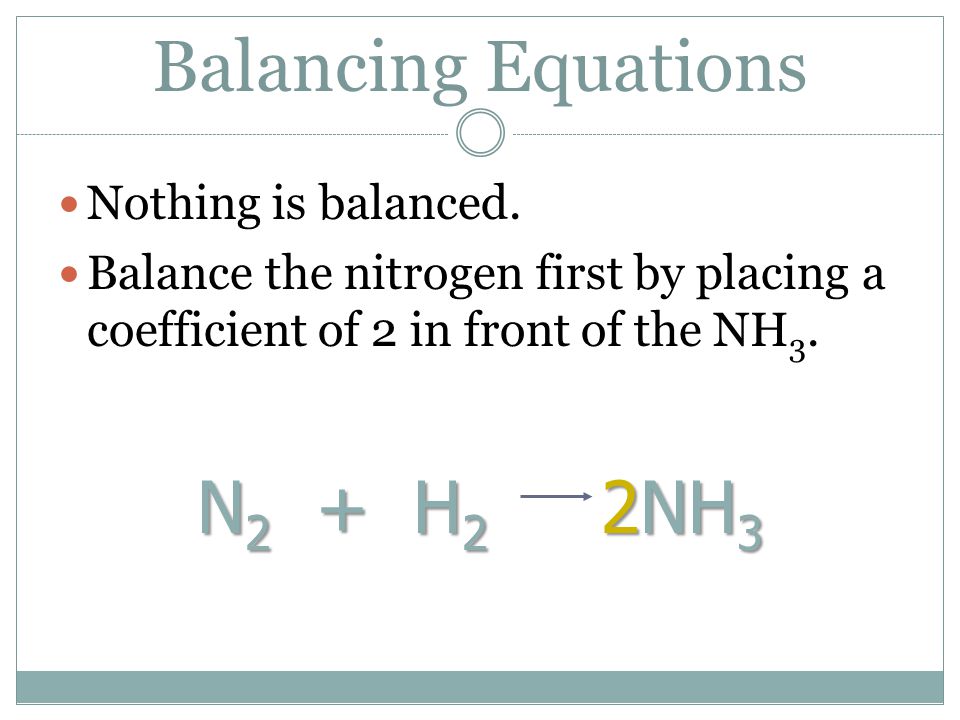
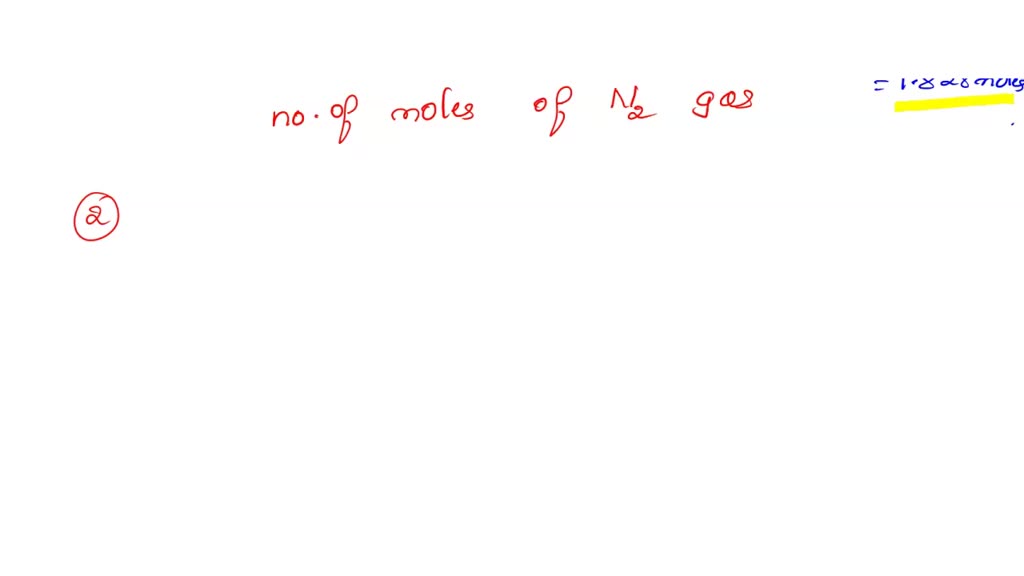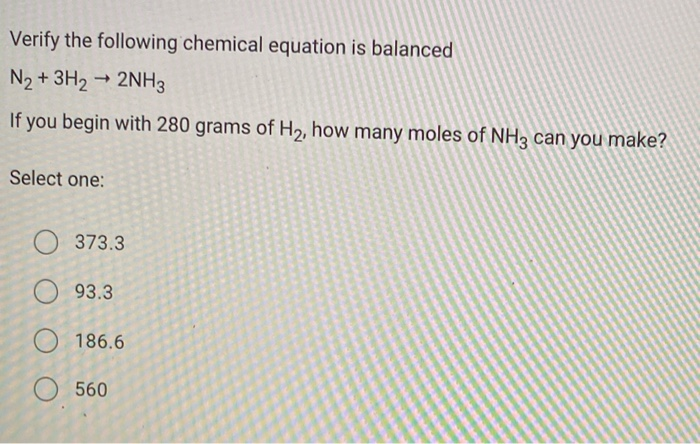⚗️2NH. N2 + 3H2 Reactants Product On the balanced equation above, how many atoms of each element are - Brainly.com

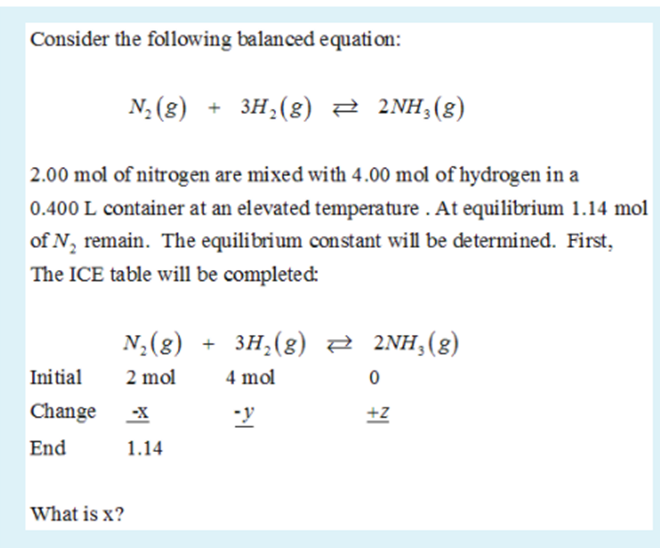
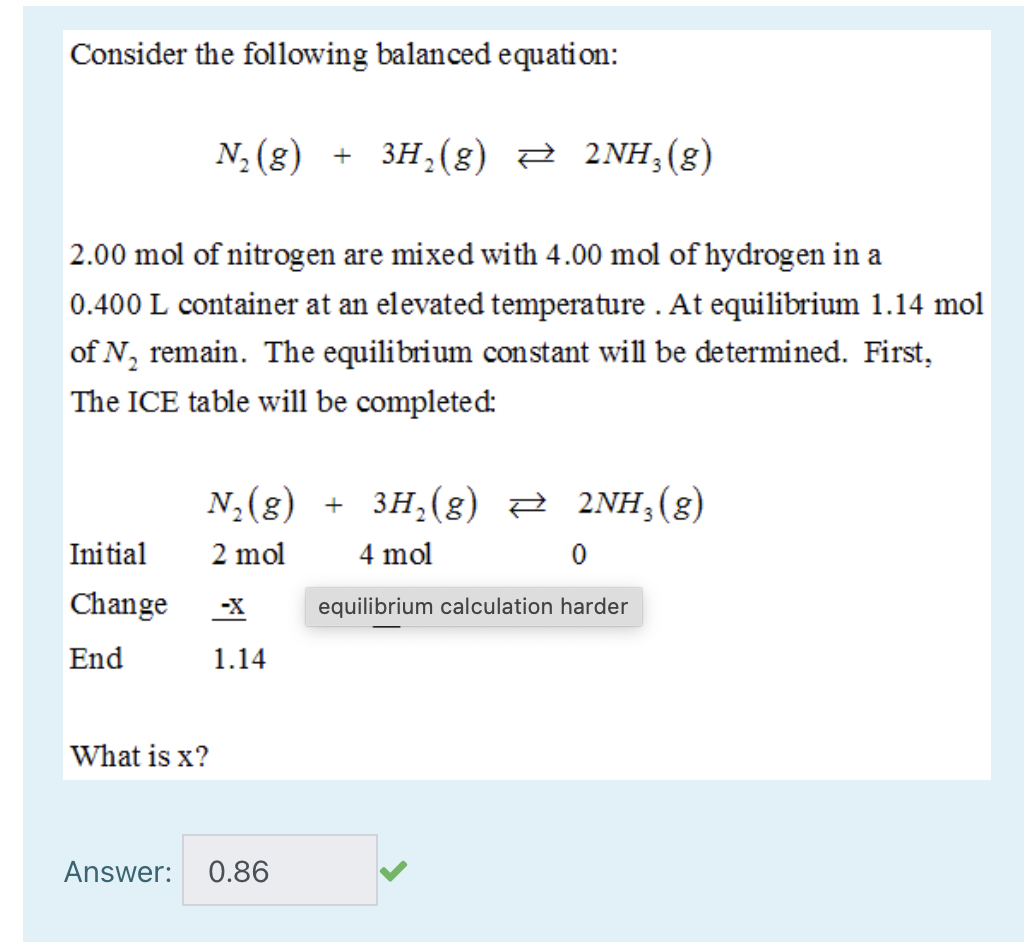
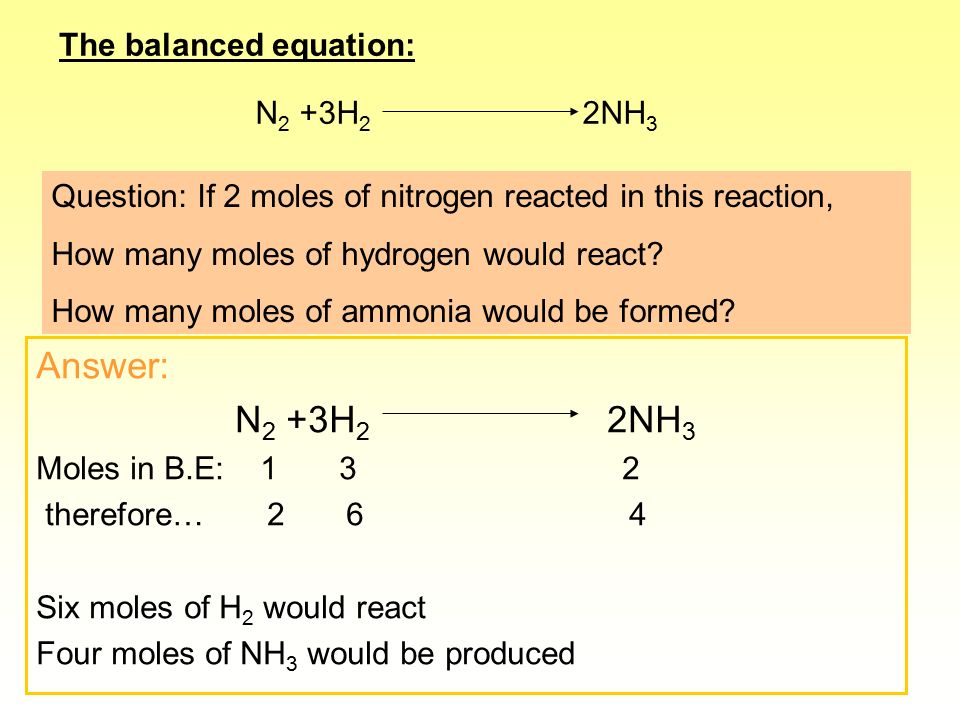
3H2(g) + N2(g)= 2NH3(g) How many moles of NH3 can be produced from 24.0 mol of H2 and excess N2 - Brainly.com

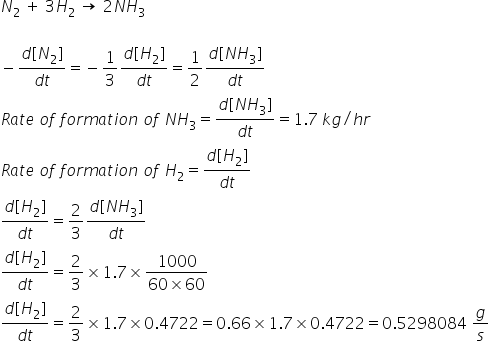
For the reaction, N2 + 3H2→ 2NH3 . The rate of change of concentration for hydrogen is 0.3 × 10^-4Ms^-1 . The rate of change of concentration ammonia is:
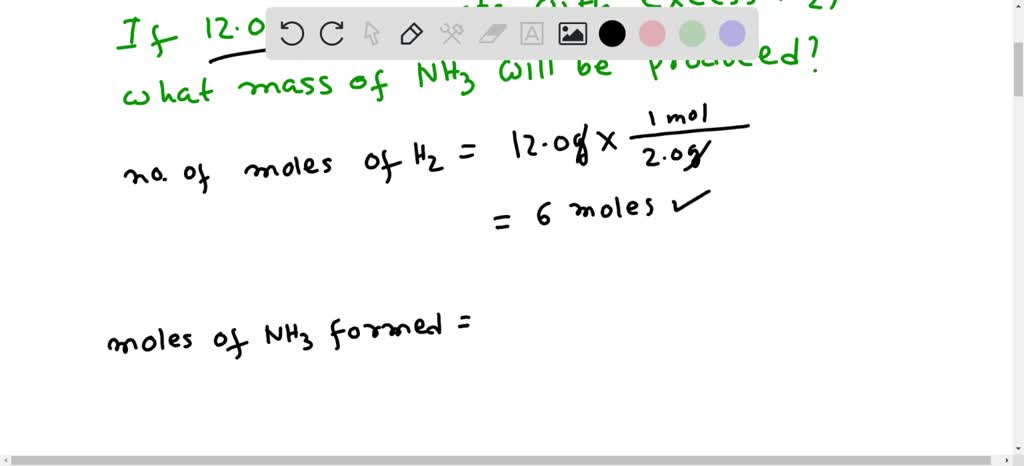
SOLVED: using the balanced equation n2 + 3h2 = 2nh3 solve if 126 grams of h2 react with excess n2 how many grams of nh3 will be produced